Sublingual immunotherapy also works in allergic cats!
Dear Colleagues,
Another month, another newsletter!
As every practitioner already knows, cats commonly present to veterinarians with pruritus and lesions of one of the following four syndromes: head-and-neck excoriations, eosinophilic diseases (e.g., eosinophilic plaques, collagenolytic granulomas, indolent ulcers), miliary dermatitis (e.g., papulo-crusted dermatitis), and self-induced (often-symmetric) alopecia. These four patterns are often attributed to an allergic etiology, and they all have been recently regrouped as manifestations of the "Feline Atopic Skin Syndrome – FASS."
For those not yet familiar with this new terminology, I recommend reading four papers on the terminology, pathogenesis, clinical signs, and treatment of FASS. These papers were generated by a subcommittee of the ICADA (International Committee on Allergic Diseases of Animals), and they can be downloaded with free access here.
The last paper of this mini-series is a systematic review that scrutinized the results of all clinical trials previously published on the treatment of this syndrome. The conclusions are noteworthy, yet not-so-surprising! The authors found satisfactory evidence for the good efficacy of oral glucocorticoids and ciclosporin, and more limited evidence for the good efficacy of topical glucocorticoids, oclacitinib, maropitant, and allergen-specific immunotherapy (ASIT; data from Table 10 therein); several other interventions were assessed as having a more moderate efficacy.
Over the years, 11 studies have reported the efficacy of ASIT for FASS, and nearly all had included cats treated with subcutaneous ASIT, either aqueous or adjuvanted.
In the February 2021 issue of the journal Veterinary Dermatology, Rubén Foj and colleagues published the full paper of their study on the clinical efficacy of sublingual immunotherapy (SLIT) in cats with skin allergies; the abstract can be found here.
This study was a prospective, multicenter, open, and uncontrolled field trial of cats with atopic dermatitis (AD). As I—and many others—will argue that cats do not spontaneously suffer from an allergic skin disease that mirrors human and canine AD, either clinically or immunologically, I will not use that term for the feline syndrome but will switch to the latest terminology.
The authors included 22 cats that had met the following criteria: 1) at least one of the typical manifestations of the FASS for more than one year, 2) a positive allergen-specific IgE serology for house dust or storage mites, and 3) all other possible causes of pruritus (including food allergy) have been ruled out.
The SLIT was formulated as an aqueous solution containing glycerinated extracts of Dermatophagoides farinae, D. pteronyssinus, Acarus siro, and/or Tyrophagus putrescentiae (LETIPharma, Barcelona, Spain), which was individualized based on the results of the serological testing (Allercept, Heska, Fribourg, Switzerland) for each cat; owners administered the SLIT once daily, as done classically.
Veterinarians were allowed to prescribe oral methylprednisolone at the time of induction of SLIT or in case of flares using a standardized protocol, and the number of tablets was accounted for during the trial.
The main outcome measures were the evolution of clinical signs (skin lesions, pruritus) using standard scales and the evolution of mite-specific IgG and IgE over time. The re-evaluations were done quarterly during the first year of SLIT.
Of the 22 cats that started the trial, three were removed as they did not finish six months of treatment; values of all other cats were taken into account for the statistical analysis.
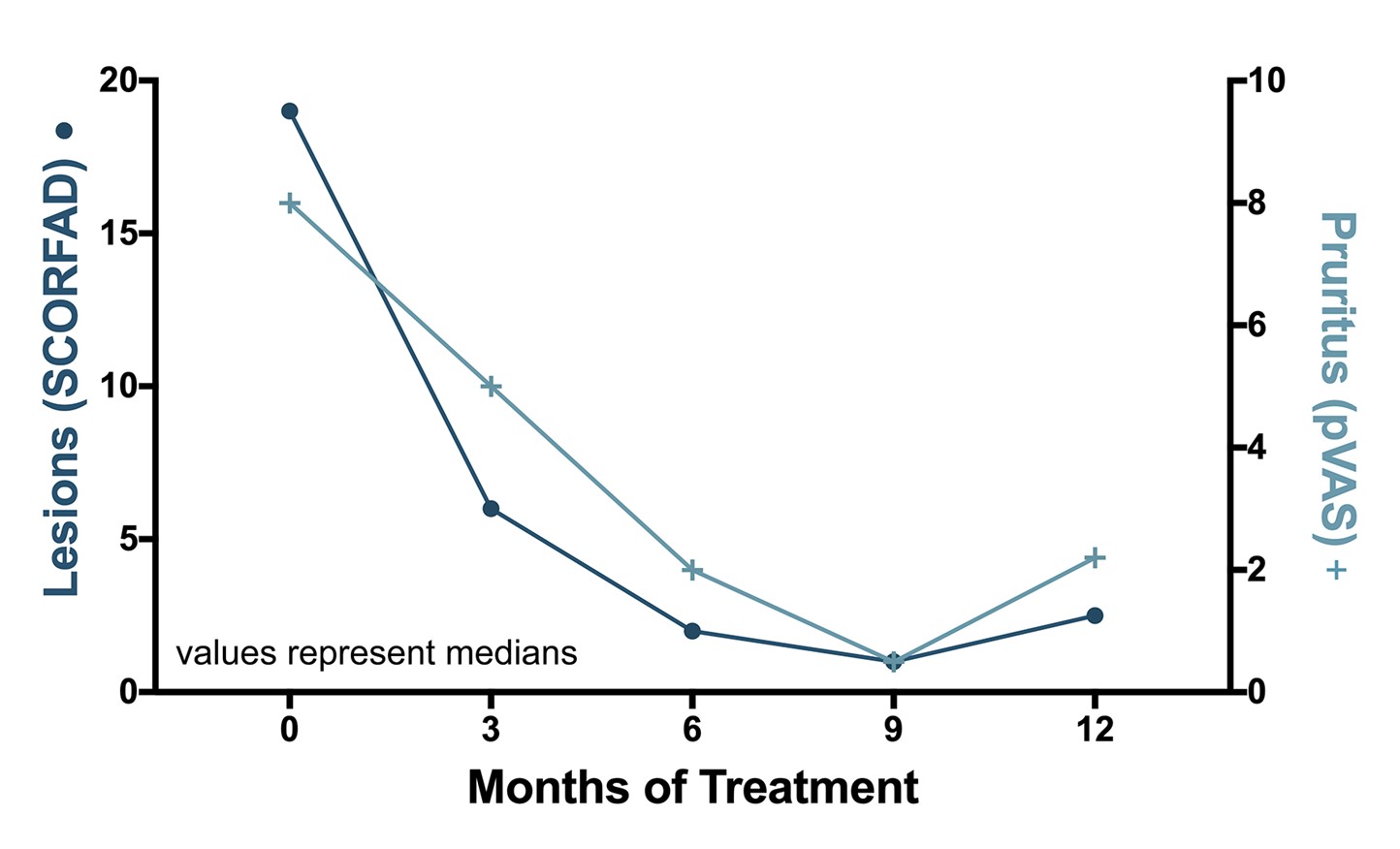
The results are noteworthy: both skin lesions and pruritus manifestations decreased impressively over time, with both scores being significantly lower as early as three months after starting treatment! (a figure adapted from the paper is shown below). Remarkably, scores for each type of the FASS's four classic presentations seemed to improve equally during the study.
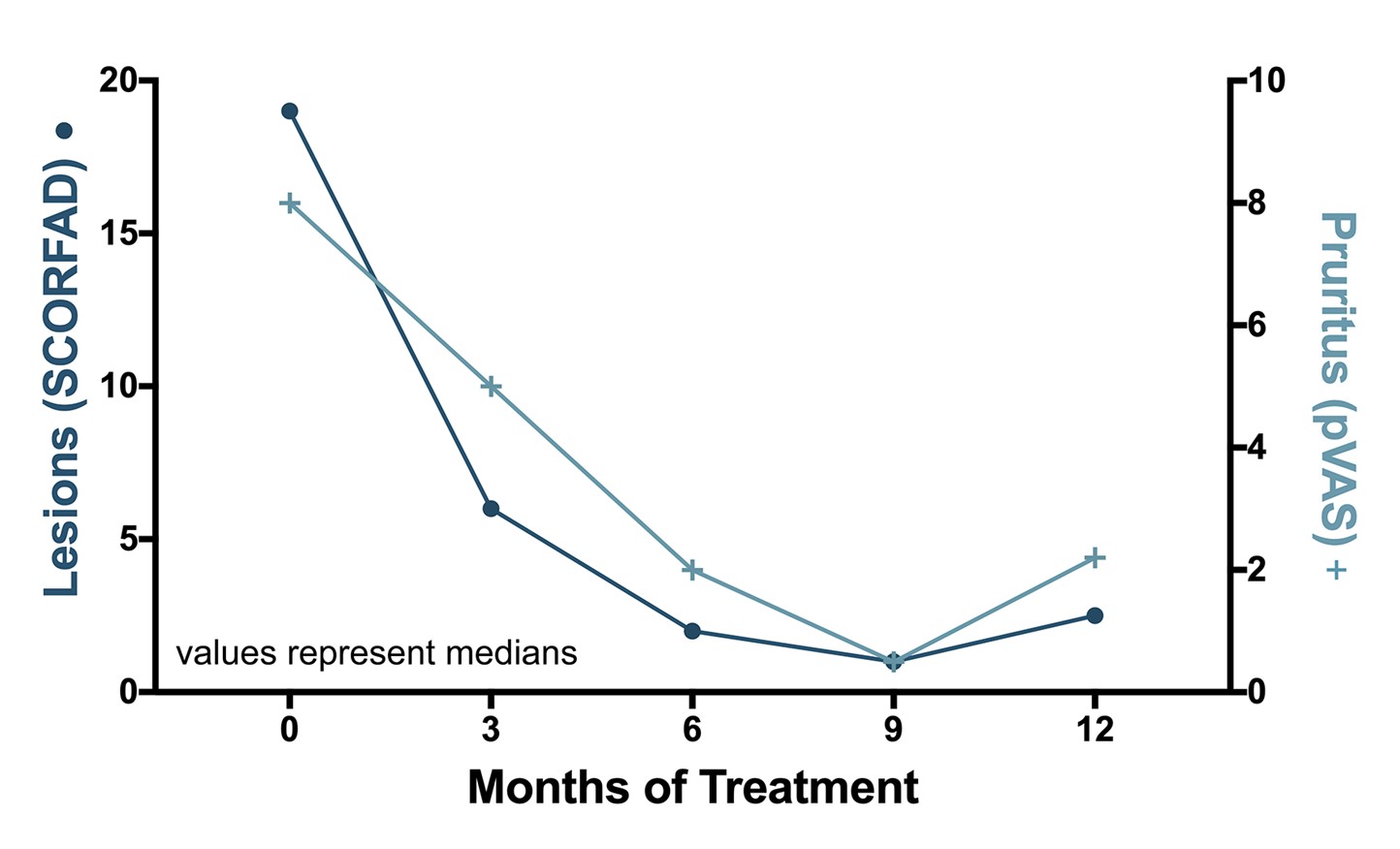
Of importance is that, at the beginning of the study, seven cats needed an average dosage of 0.8 mg/kg/day of methylprednisolone to control their signs. After three months of SLIT, only five cats needed half this dosage, and after one year, four cats needed about one-third of the original dosage.
As is usual with SLIT, adverse events were not seen.
Immunologically, it took nine months for mite-specific IgE to be lower than at baseline, while mite-specific IgG did not change appreciably.
What do I like in this study?
Despite its small size, I appreciated several aspects of this trial:
- It was the first enrolling cats with allergic dermatitis to be treated with SLIT, and,
- It used standard instruments to score skin lesions and pruritus manifestations, and,
- It allowed the use of glucocorticoids—as done in practice—but accounted simply for the reduction in the use of such medication, and,
- All clinical parameters evolved positively in unison.
What would I have liked to see?
Of course, the trial would have been of higher quality if double-blinded with a placebo-controlled group and a larger number of cats, but one could argue that the use of a placebo for a year is not ethical. Perhaps, then, a group of cats treated with ciclosporin would have been suitable controls. As outcome measures, I would have loved to see also the number (percentage) of cats with lesion and pruritus scores of normal cats at the various time points, as from the values shown on the graphs, I suspect that many cats must have been clinically normal, even early on!
Clinical bottom line:
In this small, open-label prospective trial, SLIT proved rapidly effective to reduce clinical signs and glucocorticoid needs in cats with allergic dermatitis sensitized to house dust or storage mites. As such, SLIT seems a valid option to control allergy signs in cats reluctant to be injected with the standard subcutaneous ASIT!
Till next month!
Respectfully submitted,
Thierry Olivry, DrVet, PhD, DipECVD, DipACVD
Research Professor of Immunodermatology
NC State University College of Veterinary Medicine, Raleigh, North Carolina, USA
Scientific Advisor and Dermatology & Allergy Consultant
Nextmune, Stockholm, Sweden
 Global English
Global English

 UK
UK
-Dec-21-2023-09-46-12-9148-AM.jpeg)